Chapter 1. Research Objective
1.1 Objective, Definition & Scope
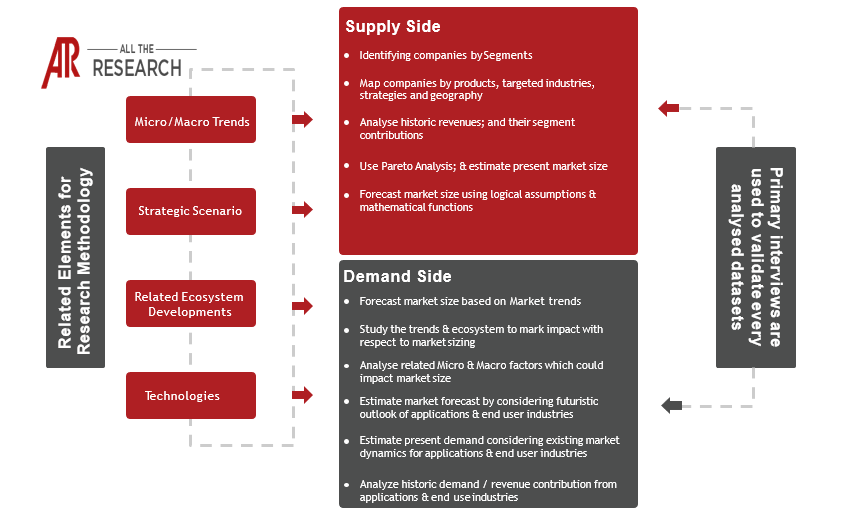
1.2 Methodology
1.2.1 Primary Research
1.2.2 Secondary Research
1.2.3 Market Forecast - Estimation & Approach
1.2.4 Assumptions & Assessments
1.3 Insights and Growth - Relevancy Mapping
1.3.1 FABRIC Platform
1.4 Data mining & efficiency
Chapter 2. Executive Summary
2.1 Vital Sign Monitoring Devices Market Overview
2.2 Interconnectivity & Related markets
2.3 Ecosystem Map
2.4 Vital Sign Monitoring Devices Market Business Segmentation
2.5 Vital Sign Monitoring Devices Market Geographic Segmentation
2.6 Competition Outlook
2.7 Key Statistics
Chapter 3. Strategic Analysis
3.1 Vital Sign Monitoring Devices Market Revenue Opportunities
3.2 Cost Optimization
3.3 Covid19 aftermath - Analyst view
3.4 Vital Sign Monitoring Devices Market Digital Transformation
Chapter 4. Market Dynamics
4.1 DROC
4.1.1 Drivers
4.1.2 Restraints
4.1.3 Opportunities
4.1.4 Challenges
4.2 PEST Analysis
4.2.1 Political
4.2.2 Economic
4.2.3 Social
4.2.4 Technological
4.3 Market Impacting Trends
4.3.1 Positive Impact Trends
4.3.2 Adverse Impact Trends
4.4 Porter's 5-force Analysis
4.5 Market News - By Segments
4.5.1 Organic News
4.5.2 Inorganic News
Chapter 5. Market Segmentation
The Vital Sign Monitoring Devices Market has been analysed to include the below segmentation:
BY PRODUCT
• STANDALONE
MONITORING DEVICES
• INTEGRATED
MONITORING DEVICES
BY PATIENT GROUP
• GERIATRIC
PATIENT GROUP
• ADULT
PATIENT GROUP
• PEDIATRIC
PATIENT GROUP
BY END-USER
• HOSPITALS
• AMBULATORY
CARE SETTINGS
• HOME CARE
SETTINGS
• OTHERS
Chapter 5A. Regional Segmentation
The Vital Sign Monitoring Devices Market has been analysed by studying the following regions:
North America
• BY PRODUCT
• BY PATIENT GROUP
• BY END-USER
Europe
• BY PRODUCT
• BY PATIENT GROUP
• BY END-USER
APAC
• BY PRODUCT
• BY PATIENT GROUP
• BY END-USER
LatAm
• BY PRODUCT
• BY PATIENT GROUP
• BY END-USER
MEA
• BY PRODUCT
• BY PATIENT GROUP
• BY END-USER
**The Regions are further studied to analyse the major countries within the respective regions. The coverage of the country level data is dynamic and is updated regularly based on the market movements. Normally, the countries covered in the report include:
• North America - United States, Canada, Mexico;
• Europe - United Kingdom, France, Italy, Germany, Spain, Rest of Europe;
• Asia Pacific - China, India, Japan, South Korea, Rest of APAC;
• Middle East & Africa - South Africa, GCC Countries, Rest of MEA;
• Latin America - Brazil, Argentina, Rest of LatAm;
Chapter 6. Market Use case studies
Chapter 7. KOL Recommendations
Chapter 8. Investment Landscape
8.1 Vital Sign Monitoring Devices Market Investment Analysis
8.2 Market M&A
8.3 Market Fund Raise & Other activity
Chapter 9. Vital Sign Monitoring Devices Market - Competitive Intelligence
9.1 Company Positioning Analysis
9.1.1 Positioning - By Revenue
9.1.2 Positioning - By Business Score
9.1.3 Legacy Positioning
9.2 Competitive Strategy Analysis
9.2.1 Organic Strategies
9.2.2 Inorganic Strategies
Chapter 10. Key Company Profiles
*The Vital Sign Monitoring Devices Market Report profiles companies based on the material impact they have on the market ecosystem. These are hence, to be read as 'Key Players' and not necessarily 'Market Leaders'.
Companies are typically profiled to include:
10.x.1 Company Fundamentals
10.x.2 Performance Overview
10.x.3 Product Overview
10.x.4 Recent Developments
Key Companies profiled in this report include:
• A&D COMPANY
• CONTEC MEDICAL SYSTEMS
• GE HEALTHCARE
• HILLROM
• KONINKLIJKE PHILIPS
• MASIMO
• MEDTRONIC
• NIHON KOHDEN
• NONIN MEDICAL
• OMRON HEALTHCARE
• OSI SYSTEMS
• SMITHS MEDICAL
• SUNTECH MEDICAL
• GENERAL MEDITECH
• CLARITY MEDICAL
• HICKS THERMOMETERS INDIA
• MEDICAL ECONET
• MICROLIFE
• MEDITECH
• SHENZHEN CREATIVE INDUSTRY
• PROGETTI
• COMDEK
• GETEMED MEDICAL AND INFORMATION TECHNOLOGY
• SHENZHEN UNICARE ELECTRONIC
• OPTO CIRCUITS (INDIA
• BEIJING CHOICE ELECTRONIC TECHNOLOGY
• SHENZHEN MINDRAY BIO-MEDICAL ELECTRONICS
• DR•GERWERK
• CARDINAL HEALTH
• COSINUSS
• BETTER LIFE MEDICAL TECHNOLOGY
• TAIDOC
• AXCENT MEDICAL
• MENNEN MEDICAL GROUP
• REMOTE DIAGNOSTIC TECHNOLOGIES
• EDAN INSTRUMENTS
Chapter 11. Appendix
11.1 About AllTheResearch (ATR)
11.2 ATR Services
11.3 Author details
11.4 Terms & Conditions
11.5 Contact us