Chapter 1. Research Objective
1.1 Objective, Definition & Scope
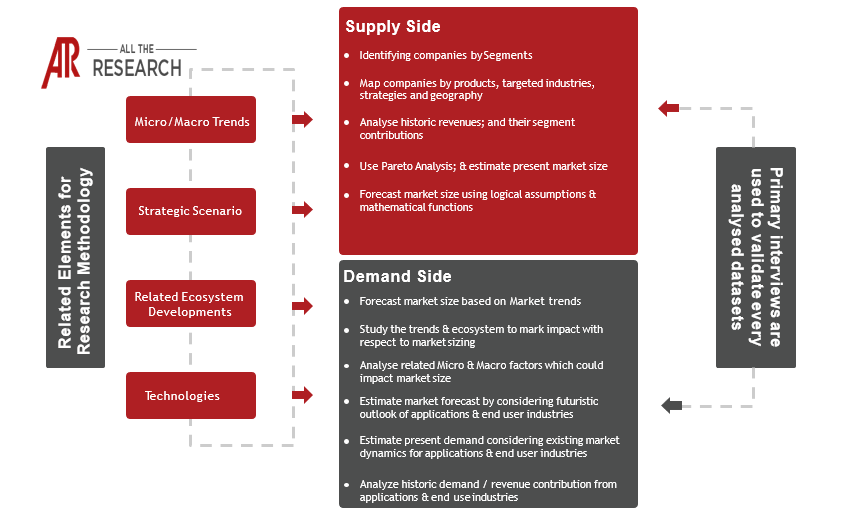
1.2 Methodology
1.2.1 Primary Research
1.2.2 Secondary Research
1.2.3 Market Forecast - Estimation & Approach
1.2.4 Assumptions & Assessments
1.3 Insights and Growth - Relevancy Mapping
1.3.1 FABRIC Platform
1.4 Data mining & efficiency
Chapter 2. Executive Summary
2.1 Lab Automation for In Vitro Diagnostics Market Overview
2.2 Interconnectivity & Related markets
2.3 Ecosystem Map
2.4 Lab Automation for In Vitro Diagnostics Market Business Segmentation
2.5 Lab Automation for In Vitro Diagnostics Market Geographic Segmentation
2.6 Competition Outlook
2.7 Key Statistics
Chapter 3. Strategic Analysis
3.1 Lab Automation for In Vitro Diagnostics Market Revenue Opportunities
3.2 Cost Optimization
3.3 Covid19 aftermath - Analyst view
3.4 Lab Automation for In Vitro Diagnostics Market Digital Transformation
Chapter 4. Market Dynamics
4.1 DROC
4.1.1 Drivers
4.1.2 Restraints
4.1.3 Opportunities
4.1.4 Challenges
4.2 PEST Analysis
4.2.1 Political
4.2.2 Economic
4.2.3 Social
4.2.4 Technological
4.3 Market Impacting Trends
4.3.1 Positive Impact Trends
4.3.2 Adverse Impact Trends
4.4 Porter's 5-force Analysis
4.5 Market News - By Segments
4.5.1 Organic News
4.5.2 Inorganic News
Chapter 5. Market Segmentation
The Lab Automation for In Vitro Diagnostics Market has been analysed to include the below segmentation:
By Equipment
• Automated Plate Handler
• Automated Liquid Handler
• Robotic Arm
• Automated Storage and Retrieval System
• Analyzer
By End User
• Academic
• Laboratory
• Other End Users
Chapter 5A. Regional Segmentation
The Lab Automation for In Vitro Diagnostics Market has been analysed by studying the following regions:
North America
• By Equipment
• By End User
Europe
• By Equipment
• By End User
APAC
• By Equipment
• By End User
LatAm
• By Equipment
• By End User
MEA
• By Equipment
• By End User
**The Regions are further studied to analyse the major countries within the respective regions. The coverage of the country level data is dynamic and is updated regularly based on the market movements. Normally, the countries covered in the report include:
• North America - United States, Canada, Mexico;
• Europe - United Kingdom, France, Italy, Germany, Spain, Rest of Europe;
• Asia Pacific - China, India, Japan, South Korea, Rest of APAC;
• Middle East & Africa - South Africa, GCC Countries, Rest of MEA;
• Latin America - Brazil, Argentina, Rest of LatAm;
Chapter 6. Market Use case studies
Chapter 7. KOL Recommendations
Chapter 8. Investment Landscape
8.1 Lab Automation for In Vitro Diagnostics Market Investment Analysis
8.2 Market M&A
8.3 Market Fund Raise & Other activity
Chapter 9. Lab Automation for In Vitro Diagnostics Market - Competitive Intelligence
9.1 Company Positioning Analysis
9.1.1 Positioning - By Revenue
9.1.2 Positioning - By Business Score
9.1.3 Legacy Positioning
9.2 Competitive Strategy Analysis
9.2.1 Organic Strategies
9.2.2 Inorganic Strategies
Chapter 10. Key Company Profiles
*The Lab Automation for In Vitro Diagnostics Market Report profiles companies based on the material impact they have on the market ecosystem. These are hence, to be read as 'Key Players' and not necessarily 'Market Leaders'.
Companies are typically profiled to include:
10.x.1 Company Fundamentals
10.x.2 Performance Overview
10.x.3 Product Overview
10.x.4 Recent Developments
Key Companies profiled in this report include:
• Cognex Corporation
• Roche Holding AG
• Thermo Fisher Scientific Inc.
• Danaher Corporation
• Agilent Technologies Inc.
• Abbott Laboratories
• PerkinElmer Inc.
• Tecan Group Ltd
• Becton, Dickinson and Company
• Siemens Healthineers AG
Chapter 11. Appendix
11.1 About AllTheResearch (ATR)
11.2 ATR Services
11.3 Author details
11.4 Terms & Conditions
11.5 Contact us