Market Definition:
In-vitro diagnostics are medical examinations that assist doctors to diagnose a patient’s condition and provide appropriate treatments. Blood, tissue, saliva or other samples from a human’s body are collected which on analyzing can help detect infections, diseases and other conditions. Since in-vitro means in-glass, these tests are conducted in either test tubes or similar equipments. The samples can be collected at laboratories, healthcare facilities or at home while the test is generally performed in specialized centers like laboratories and hospitals where adequate equipments are available.
Market Size:
The Global in vitro diagnostic market size was US$65147.91 Million in 2019 and is expected to reach US$121864.93 Million by 2030; This converts into a compounded annual growth rate (CAGR) of 5.9% for the forecast period.
Market Overview:
The pandemic has created an upsurge in the in-vitro diagnostic market. Testing is a crucial factor for the diagnosis of the disease. With an increasing number of cases globally, the market has seen a sharp rise due to continuous testing. Rising demand for IVD kits and reagents for rapid diagnosis has significantly contributed to the expansion of the market. Moreover, the convenience and safety that IVDs offer such as a collection of samples in-home care settings has been one of the major influencing factors for the expansion of the market even though most industries have been negatively impacted due to the pandemic.
The Global In-Vitro Diagnostic Market is segmented to include
By Product:
By Test Type:
By Application:
By End-User
On the basis of product, the in-vitro diagnostic market is divided into – instruments, consumable, kits, reagents and software. The consumables segment dominated the market capturing more than 20%. The factors responsible for the lead of this segment are rising need to identify diseases, growing use of kits and reagents and low cost. The kits segment is projected to be the fastest-growing among the different product types. It is estimated to expand at a compound annual growth rate of over 6%.

The different test types of the in-vitro diagnostic market are immunoassay, clinical chemistry, molecular diagnostics, whole blood glucose monitoring, microbiology, hematology, anatomic pathology, coagulation, critical care, urinalysis and others. Molecular diagnostics segment is anticipated to expand at the highest growth rate of over 8.5% during the forecast timeline. The benefits of this segment such as early detection of cancer, infectious diseases and genetic disorders at prenatal stage propels the segment forward. However, currently, the immunoassay segment is leading the market garnering close to 35%. Factors like the increasing prevalence of chronic diseases such as H.I.V. and its ability to accurately detect infectious microbes have ensured the dominance of the segment.
Among its widespread applications, the in-vitro diagnostic market was led by the cardiology & blood disorders segment. Alcohol abuse, smoking, unhealthy diets, reduced physical activity, etc. have resulted in an increased number of cardiovascular diseases. Additionally, diabetes that requires continuous monitoring is driving the growth of the segment. As the result, the cardiology & blood disorders segment accounted for over 20% of the market share. The infectious disease segment, on the other hand, is projected to grow at a compound annual growth rate of nearly 7%. Increasing incidences of pneumonia, HIV, AIDS, etc. is thrusting the segment forward.
Finally classified on the basis of end-user, the hospital segment held almost 40% of the in-vitro diagnostic market in 2019. This can be attributed to the collaboration of diagnostic centers and hospitals and rise in hospitalization across the globe. Also, improvement in existing healthcare infrastructure and development of new is predicated to enhance the segments hold over the global market. All of these factors combined are generating huge demand for in-vitro diagnostic testing. Besides hospitals, clinics are now collaborating with diagnostic laboratories for commercial advantages. The number of people frequently visiting clinics is steadily growing. Therefore, the clinic segment is anticipated to register a growth rate of approximately 9% over the forecast period.
The market is studied by analyzing all the key market participants across the ecosystem. The key players in the global in-vitro diagnostic market include, but not limited to:
Market Geography Overview: Based on geography market is segmented as below:
North America held the largest share of the in-vitro diagnostic market in 2019. It is predicted to dominate the market throughout the forecast timeline. Rising number of diseases coupled with increased awareness is positively influencing the growth of the market in the region. Furthermore, the presence of well-established players is ensuring the market expansion. Advancing technology, ease in device availability, a well-penetrated healthcare system and various supportive government initiatives are other factors affecting the regional market progress.
Asia – Pacific is estimated to expand at the fastest rate over the forecast period. Growing population, rapid urbanization and changing lifestyle patterns has increased the prevalence of numerous diseases in the region. This, in turn, raises the demand for in-vitro diagnostic tools significantly. Increase in disposable incomes, economic stability in the region and advancing healthcare is steadily developing the in-vitro diagnostic market.
The European in-vitro diagnostic market is envisaged to grow owing to the rising healthcare expenditure and increasing adoption of PoC testing devices. The Middle East and African region will witness a growth due to the growing healthcare infrastructure and entry of global players in the region.

In-Vitro Diagnostic Market Dynamics:
A) Drivers:
Growing demand from patients for a diagnostic test that are non-invasive and provide results without having to wait to for too long is boosting the development of the in-vitro diagnostic market especially in emerging countries.
In the United States, the population of elderly people was around 50 million in 2019. In Japan, the elderly accounted for more than a quarter of the total population while the same was over 20% in Germany. The prevalence of age associated disorders/diseases increases directly in proportion with the geriatric population. Therefore, the growing geriatric population is expected to drive the in-vitro diagnostic market forward.
A large number of people from the European region suffer due to pneumonia. Furthermore, nearly 1 million people in the U.S. undergo treatment for this chronic condition. Besides this, diabetes is another chronic disease that is largely affecting majority of the population. Rising number of cases of such chronic diseases and infectious diseases will significantly expand the in-vitro diagnostic market.
Economic stability, rising income levels and increased awareness among individuals has resulted in growing investment on healthcare. Demand for improved healthcare facilities is generating better testing technologies which, in turn, would help expand the in-vitro diagnostic market.
Adoption of rapid and accurate tests for diagnosis is positively affecting the in-vitro diagnostic market. However, the increasing demand for PoC devices is projected to boost the market growth. Technological advancements in this area are expected to accelerate the market progress. Benefits such as easy access to these tests and quick test results are propelling the market expansion.
B) Restraints
Developments in the reimbursements schemes adversely affect the in-vitro diagnostic market. For instance, the revision in the reimbursement mechanism by Medicare for few IVD tests have reduced the reimbursement rates by nearly 75%. This, in turn, deters patients and severely affects the market growth.
Legal and regulatory requirements specific to in-vitro diagnostics are becoming more stringent especially in the North American and European region. This poses challenges for several manufacturers and also discourages small and medium enterprises from entering into the market. Therefore, an uncertain or continuously changing regulatory environment may restrict the in-vitro diagnostic market growth.
C) Opportunities
Rise in demand for molecular diagnostics and an epigenomics-based approach are expected to create numerous opportunities for the growth of the in-vitro diagnostic market.
Recent News and Developments in the market:

Ask for free product review call with the author

Share your specific research requirements for a customized report
Request for due diligence and consumer centric studies

Request for study updates, segment specific and country level reports
Chapter 1. Research Objective
1.1 Objective, Definition & Scope
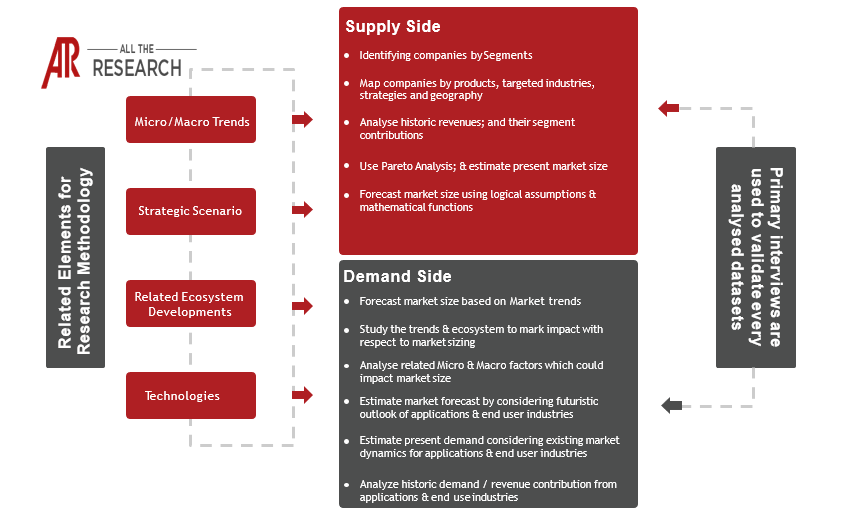
1.2 Methodology
1.2.1 Primary Research
1.2.2 Secondary Research
1.2.3 Market Forecast - Estimation & Approach
1.2.4 Assumptions & Assessments
1.3 Insights and Growth - Relevancy Mapping
1.3.1 FABRIC Platform
1.4 Data mining & efficiency
Chapter 2. Executive Summary
2.1 Global In Vitro Diagnostic Market Overview
2.2 Interconnectivity & Related markets
2.3 Ecosystem Map
2.4 Global In Vitro Diagnostic Market Business Segmentation
2.5 Global In Vitro Diagnostic Market Geographic Segmentation
2.6 Competition Outlook
2.7 Key Statistics
Chapter 3. Strategic Analysis
3.1 Global In Vitro Diagnostic Market Revenue Opportunities
3.2 Cost Optimization
3.3 Covid19 aftermath - Analyst view
3.4 Global In Vitro Diagnostic Market Digital Transformation
Chapter 4. Market Dynamics
4.1 DROC
4.1.1 Drivers
4.1.1.1 Rise in Adoption of Rapid, Minimally Invasive and
Non-Invasive Diagnostics Tools & Techniques
4.1.1.2 Rise in the Global Geriatric Population
4.1.1.3 High Number of Patients with Infectious and Chronic Diseases
4.1.1.4 Rise in the Global Healthcare Expenditure
4.1.1.5 Rise in the Demand for POC Testing
4.1.2 Restraints
4.1.2.1 Uneven Reimbursement Scenario
4.1.2.2 Uncertain Regulatory Environment
4.1.3 Opportunities
4.1.3.1 Rise in the Demand for Molecular Diagnostics
4.1.3.2 Epigenomics-Based Approach
4.1.4 Challenges
4.2 PEST Analysis
4.2.1 Political
4.2.2 Economic
4.2.3 Social
4.2.4 Technological
4.3 Market Impacting Trends
4.3.1 Positive Impact Trends
4.3.2 Adverse Impact Trends
4.4 Porter's 5-force Analysis
4.5 Market News - By Segments
4.5.1 Organic News
4.5.2 Inorganic News
4.6 Price Trend Analysis
Chapter 5. Market Segmentation
5.1 The Global In Vitro Diagnostic Market Segmentation by Product - Forecast till 2027
5.1.1 Instruments
5.1.2 Consumables
5.1.3 Kits
5.1.4 Reagents
5.1.5 Software
5.2 The Global In Vitro Diagnostic Market Segmentation by Test Type - Forecast till 2027
5.2.1 Immunoassays
5.2.2 Clinical Chemistry
5.2.3 Molecular Diagnostics
5.2.4 Whole Blood Glucose Monitoring
5.2.5 Microbiology
5.2.6 Hematology
5.2.7 Anatomic Pathology
5.2.8 Coagulation
5.2.9 CriticalCare
5.2.10 Urinalysis
5.2.11 Others (Allergen and Endocrinology testing)
5.3 The Global In Vitro
Diagnostic Market Segmentation by Application - Forecast till 2027
5.3.1 Infectious Diseases
5.3.2 Cardiology and Blood Disorders
5.3.3 Oncology/Cancer
5.3.4 Immune Diseases
5.3.5 Diabetes
5.3.6 Drug Testing
5.3.7 Women Health
5.3.8 Nephrology
5.3.9 HIV/AIDS
5.3.10 Others (blood donor screening, and human antigen testing)
5.4 The Global In Vitro Diagnostic Market Segmentation by End User - Forecast till 2027
5.4.1 Hospitals
5.4.2 Central Laboratories
5.4.3 Point-of-Care (POC)
5.4.4 Clinics
5.4.5 Academic Institutes
5.4.6 Others (diagnostic centers, and CROs)
Chapter 5 A. Regional Forecast
North America Global In Vitro Diagnostic Market Segmentation
Europe Global In Vitro Diagnostic Market Segmentation
APAC Global In Vitro Diagnostic Market Segmentation
Latin America (LATAM) Global In Vitro Diagnostic Market Segmentation
Middle East & Africa (MEA) Global In Vitro Diagnostic Market Segmentation
**The Regions are further studied to analyze the major countries within the respective regions. The coverage of the country level data is dynamic and is updated regularly based on the market movements. Normally, the countries covered in the report include:
North America - United States, Canada, Mexico; Europe - United Kingdom, France, Italy, Germany, Spain, Rest of Europe; Asia Pacific - China, India, Japan, South Korea, Rest of APAC; Middle East & Africa - South Africa, GCC Countries, Rest of MEA; LatAm - Brazil, Argentina, Rest of LatAm;
Chapter 6. Market Use case studies
Chapter 7. KOL Recommendations
Chapter 8. Investment Landscape
8.1 Global In Vitro Diagnostic Market Investment Analysis
8.2 Market M&A
8.3 Market Fund Raise & Other activity
Chapter 9. Global In Vitro Diagnostic Market - Competitive Intelligence
9.1 Company Positioning Analysis
9.1.1 Positioning - By Revenue
9.1.2 Positioning - By Business Score
9.1.3 Legacy Positioning
9.2 Competitive Strategy Analysis
9.2.1 Organic Strategies
9.2.2 Inorganic Strategies
Chapter 10. Key Company Profiles
*The Global In Vitro Diagnostic Market Report profiles companies based on the material impact they have on the market ecosystem. These are hence, to be read as 'Key Players' and not necessarily 'Market Leaders'.
Companies are typically profiled to include:
10.x.1 Company Fundamentals
10.x.2 Performance Overview
10.x.3 Product Overview
10.x.4 Recent Developments
The Companies profiled in this Report include:
Chapter 11. Appendix
11.1 About ResearchCMFE
11.2 Service offerings
11.3 Author details
11.4 Terms & Conditions
11.5 Contact us