The global radioimmunotherapy market was valued at US$ 81.9 Mn in 2018 and is expected to reach US$ 132.7 Mn by the year 2026, growing at a CAGR of 6.5% during the forecast period. The market is still in its nascent stages as there is only one approved product.
However, the emerging clinical pipeline of radioimmunotherapy looks promising and will create opportunities for market. Radioimmunotherapy is the combination of radiation therapy and immunotherapy. In this, a monoclonal antibody (mAb) is paired with a radiotracer (radioactive material). When injected, radio labeled antibody binds to the specific cancer cell which is destroyed by its radioactivity. Enhanced tumor recognition and immune cell targeting is the one of the key benefits of this therapy. Also, as the radiation is localized to the tumour site, risk of damage to the surrounding healthy tissue is reduced. Increasing prevalence of cancer and its mutation forms, adverse events with radiation therapy, need for targeted therapy, and high immune specificity through monoclonal antibodies are the key drivers of Radioimmunotherapy market.
Radioimmunotherapy Market Segmentation |
|
|
By Drug Type |
|
|
By Procedure Type |
|
|
By Indication |
|
|
By Radioisotope |
|
|
By End-user |
|
|
By Region |
|
The expected regulatory approval of multiple products across diverse cancer indications is likely to increase, and affordable pricing due to benefit of insurance coverage are set to create more opportunities and increase use. The key players operating in the Radioimmunotherapy market are Bayer AG, Actinium Pharmaceuticals, Acrotech Ltd, Nordic Nanovector and Orano Med amongst others. Mergers and acquisitions and strategic partnerships with key players across the value chain are some of the key strategies adopted by companies to sustain in the market.

By Drug Type, Ibritumomab segment held the largest share of Radioimmunotherapy Market in the year 2018
Ibritumomab is a CD-20 monoclonal antibody that is used for the treatment of relapsed or refractory B-cell non-Hodgkin's lymphoma. It is conjugated to Yttrium-90 and is the only approved radioimmunotherapy drug.
Ibritumomab is a murine derived antibody that has a short half-life in serum, which is an important characteristic for reducing bone marrow toxicity of the attached radioisotope. This is the main reason for which it was selected as an antibody for Zevalin over other mAbs. Although Zevalin drug achieved an overall 74% response rate and complete response in around 15% patients, the product has witnessed decreasing sales over the years. Due to this reason, there has been continuous change in the ownership of this product. In March 2019, Acrotech Pharmaceuticals acquired Zevalin from Spectrum Pharmaceuticals.
By Procedure Type, beta emitting radioimmunotherapy shows held the largest market share in the year 2018
Based on the procedure type, the beta emitting radioimmunotherapy market accounted for a share of 95.0% in 2018. Beta emitting radionuclides have long path length which can offer adequate radiation to cancer cells. Apart from this, beta emitting radionuclides provide uniform radiation dose to the tumor cells and are suitable for treatment of solid tumors or large tumors.
The demand for alpha emitting radionuclides for radioimmunotherapy is also anticipated to remain high in the coming years.
North America is dominating the global Radioimmunotherapy market
North America holds largest market share in the radioimmunotherapy market followed by Europe in 2018. The geographical analysis shows the radioimmunotherapy is at the stage growing in major developed such as US, Germany, UK etc. The major factors are infrastructure for production of isotopes, availability of radioactive materials and novel technologies to perform the treatment itself.
In developing countries such as China, India, and Brazil, radioimmunotherapy is still in its nascent stages and therefore the share of Asia Pacific and Rest of the World region is expected to remain low during the forecast period.
Company Profiles and Competitive Intelligence
The key players operating inRadioimmunotherapy market are Bayer AG, Actinium Pharmaceuticals, Acrotech Ltd, Nordic Nanovector and Orano Med amongst others. Mergers and acquisitions and strategic partnerships with key players across the value chain are some of the key strategies adopted by companies to sustain in the market.

Ask for free product review call with the author

Share your specific research requirements for a customized report
Request for due diligence and consumer centric studies

Request for study updates, segment specific and country level reports
Table of Content
Chapter 1 Preface
1.1 Report Description
1.1.1 Procedure Type of the Report
1.1.2 Target Audience
1.1.3 USP and Key Offerings
1.2 Research Scope
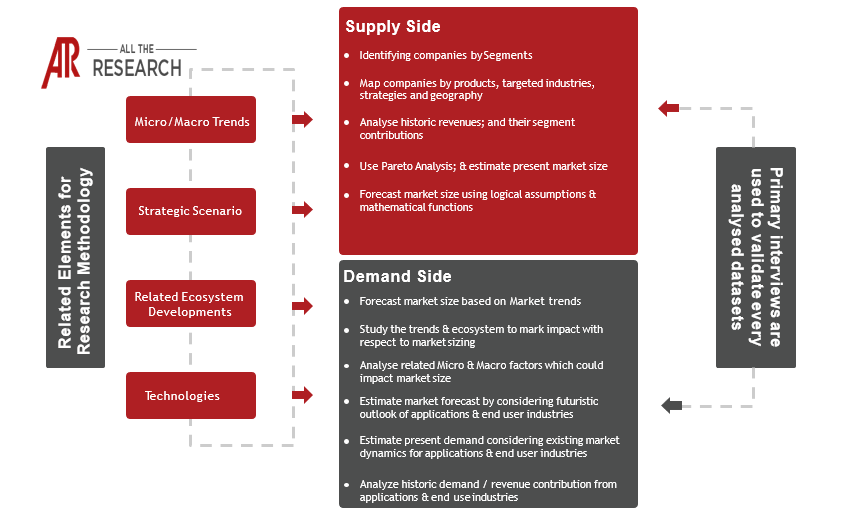
1.3 Research Methodology
1.3.1 Phase I – Secondary Research
1.3.2 Phase II – Primary Research
1.3.3 Phase III – Expert Panel Review
1.3.4 Approach Adopted
1.3.4.1 Top-Down Approach
1.3.4.2 Bottom-Up Approach
1.3.5 Assumptions
1.4 Market Segmentation Scope
Chapter 2 Executive Summary
2.1 Market Summary
2.1.1 Global Radioimmunotherapy Market, 2016-2026, (US$ Mn)
2.1 Market Snapshot: Global Radioimmunotherapy Market
2.2 Market Dynamics
2.3 Global Radioimmunotherapy Market, by Segment, 2018
2.3.1 Global Radioimmunotherapy Market, by Drug Type, 2018, (US$ Mn)
2.3.3 Global Radioimmunotherapy Market, by Procedure Type, 2018 (US$ Mn)
2.3.4 Global Radioimmunotherapy Market, by Indication, 2018 (US$ Mn)
2.3.5 Global Radioimmunotherapy Market, by Radioisotope, 2018 (US$ Mn)
2.3.6 Global Radioimmunotherapy Market, by End-user, 2018 (US$ Mn)
2.3.7 Global Radioimmunotherapy Market, by Region, 2018 (US$ Mn)
2.4 Premium Insights
2.4.1 Radioimmunotherapy Process
2.4.1.1 Equipment
2.4.1.2 Preparation
2.4.1.3 Operation and more
2.4.2 Price Analysis
2.4.3 Regulations and Treatment Guidelines
2.4.4 Developed vs. Developing Economies, 2018 vs. 2026
2.4.5 Supply Chain Analysis and Raw Materials
2.4.6 Emerging Global Pipeline
2.4.7 Technology Analysis (Including Manufacturing)
2.4.8 Unmet Needs and Gap Analysis
2.4.9 Key Stakeholder analysis
2.4.10 Reimbursement Scenario in Key markets
2.4.11 Patent Analysis
2.4.12 Association
2.4.13 Literature review
Chapter 3 Market Dynamics
3.1 Market Overview
3.2 Market Drivers
3.2.1 Rising prevalence of cancer indications, especially with mutations that are resistant to conventional treatment
3.2.2 Successful regulatory approvals and establishing treatment efficacy of
3.2.3 Growing awareness on clinical benefits of immunotherapy in combination with radiotherapy
3.2.4 Potential for Radiation therapy to overcome resistance to immune checkpoint blockade
3.2.5 Treatment targeted to the actual malignant tissue without damaging the surrounding healthy tissues
3.3 Market Challenges
3.3.1 Challenges with maximizing clinical efficacy of this promising combination
3.3.2 Concerns on aspects of clinical non-response
3.3.3 Reimbursement concerns in the key markets
3.3.4 Higher risk of immune mediated side effects as compared to individual therapy
3.3.5 High cost of therapy
3.4 Market Opportunities
3.4.1 Growing prospects for improved clinical efficacy of this combination
3.4.2 Anticipated regulatory approval for multiple products across diverse cancer indications
3.4.3 Affordable pricing and benefit of insurance coverage
3.4.4 Individualizing patient treatment and minimizing side effects using quantitative methods for estimating the radiation-absorbed dose for both tumour tissue and normal tissue
3.5 Industry SWOT Analysis
3.6 Porter’s Five Forces Analysis
Chapter 4 Global Radioimmunotherapy Market, by Drug Type
4.1 Market Overview, by Drug Type
4.1.1 Global Radioimmunotherapy Market, by Drug Type, 2016-2026 (US$ Mn)
4.1.2 Incremental Opportunity, by Drug Type, From 2018-2026
4.2 Tositumomab
4.2.1 Global Radioimmunotherapy Market, by Tositumomab , 2016-2026, (US$ Mn)
4.3 Apamistamab
4.3.1 Global Radioimmunotherapy Market, by Apamistamab, 2016-2026, (US$ Mn)
4.4 Epratuzumab
4.4.1 Global Radioimmunotherapy Market, by Epratuzumab, 2016-2026, (US$ Mn)
4.5 Ibritumomab
4.5.1 Global Radioimmunotherapy Market, by Ibritumomab, 2016-2026, (US$ Mn)
4.6 Streptavidin Fusion Protein
4.6.1 Global Radioimmunotherapy Market, by Streptavidin Fusion Protein, 2016-2026, (US$ Mn)
4.7 Lilotomab
4.7.1 Global Radioimmunotherapy Market, by Lilotomab, 2016-2026, (US$ Mn)
4.8 Omburtamab
4.8.1 Global Radioimmunotherapy Market, by Omburtamab, 2016-2026, (US$ Mn)
4.9 Others
4.9.1 Global Radioimmunotherapy Market, by Others, 2016-2026, (US$ Mn)
Chapter 5 Global Radioimmunotherapy Market, by Procedure Type
5.1 Market Overview, by Procedure Type
5.1.1 Global Radioimmunotherapy Market, by Procedure Type, 2016-2026 (US$ Mn)
5.1.2 Incremental Opportunity, by Procedure Type, From 2018-2026
5.2 Alpha Emission
5.2.1 Global Radioimmunotherapy Market, by Alpha Emission, 2016-2026, (US$ Mn)
5.3 Beta Emission
5.3.1 Global Radioimmunotherapy Market, by Beta Emission, 2016-2026, (US$ Mn)
Chapter 6 Global Radioimmunotherapy Market, by Indication
6.1 Market Overview, by Indication
6.1.1 Global Radioimmunotherapy Market, by Indication, 2016-2026 (US$ Mn)
6.1.2 Incremental Opportunity, by Indication, From 2018-2026
6.2 Leukemia
6.2.1 Global Radioimmunotherapy Market, by Leukemia, 2016-2026, (US$ Mn)
6.3 Lymphoma
6.3.1 Global Radioimmunotherapy Market, by Lymphoma, 2016-2026, (US$ Mn)
6.4 Breast cancer
6.4.1 Global Radioimmunotherapy Market, by Breast cancer, 2016-2026, (US$ Mn)
6.5 Lung cancer
6.5.1 Global Radioimmunotherapy Market, by Lung cancer, 2016-2026, (US$ Mn)
6.6 Prostate cancer
6.6.1 Global Radioimmunotherapy Market, by Prostate cancer, 2016-2026, (US$ Mn)
6.7 Others
6.7.1 Global Radioimmunotherapy Market, by Others, 2016-2026, (US$ Mn)
Chapter 7 Global Radioimmunotherapy Market, by Radioisotope
7.1 Market Overview, by Radioisotope
7.1.1 Global Radioimmunotherapy Market, by Radioisotope, 2016-2026 (US$ Mn)
7.1.2 Incremental Opportunity, by Radioisotope, From 2018-2026
7.2 Yttrium-90
7.2.1 Global Radioimmunotherapy Market, by Yttrium-90 , 2016-2026, (US$ Mn)
7.3 Iodine-131
7.3.1 Global Radioimmunotherapy Market, by Iodine-131, 2016-2026, (US$ Mn)
7.4 Thorium-227
7.4.1 Global Radioimmunotherapy Market, by Thorium-227, 2016-2026, (US$ Mn)
7.5 Bismuth-212 (Lead-212)
7.5.1 Global Radioimmunotherapy Market, by Bismuth-212 (Lead-212), 2016-2026, (US$ Mn)
7.6 Actinium-225
7.6.1 Global Radioimmunotherapy Market, by Actinium-225, 2016-2026, (US$ Mn)
7.7 Lutetium-177
7.7.1 Global Radioimmunotherapy Market, by Lutetium-177, 2016-2026, (US$ Mn)
7.8 Others
7.8.1 Global Radioimmunotherapy Market, by Others, 2016-2026, (US$ Mn)
Chapter 8 Global Radioimmunotherapy Market, by End-user
8.1 Market Overview, by End-user
8.1.1 Global Radioimmunotherapy Market, by End-user, 2016-2026 (US$ Mn)
8.1.2 Incremental Opportunity, by End-user, From 2018-2026 64
8.2 Hospitals
8.2.1 Global Radioimmunotherapy Market, by Hospitals, 2016-2026, (US$ Mn)
8.3 Physician office and Physician Group Practices
8.3.1 Global Radioimmunotherapy Market, by Physician office and Physician Group Practices, 2016-2026, (US$ Mn)
8.4 Drug R&D
8.4.1 Global Radioimmunotherapy Market, by Drug R&D, 2016-2026, (US$ Mn)
Chapter 9 Global Radioimmunotherapy Market, by Region
9.1 Market Overview, by Region
9.1.1 Global Radioimmunotherapy Market, by Region, 2016-2026, (US$ Mn)
9.2 Attractive Investment Opportunity, by Region, 2018
9.3 North America Radioimmunotherapy Market
9.3.1 North America Radioimmunotherapy Market, by Drug Type, 2016-2026 (US$ Mn)
9.3.2 North America Radioimmunotherapy Market, by Procedure Type, 2016-2026 (US$ Mn)
9.3.1 North America Radioimmunotherapy Market, by Indication, 2016-2026 (US$ Mn)
9.3.2 North America Radioimmunotherapy Market, by Radioisotope, 2016-2026 (US$ Mn)
9.3.3 North America Radioimmunotherapy Market, by End-user, 2016-2026 (US$ Mn)
9.3.4 United States Country Profile
9.3.4.1 United States Radioimmunotherapy Market, 2016-2026 (US$ Mn)
9.3.5 Canada Country Profile
9.3.5.1 Canada Radioimmunotherapy Market, 2016-2026 (US$ Mn)
9.4 Europe Radioimmunotherapy Market
9.4.1 Europe Radioimmunotherapy Market, by Drug Type, 2016-2026 (US$ Mn)
9.4.2 Europe Radioimmunotherapy Market, by Procedure Type, 2016-2026 (US$ Mn)
9.4.3 Europe Radioimmunotherapy Market, by Indication, 2016-2026 (US$ Mn)
9.4.4 Europe Radioimmunotherapy Market, by Radioisotope, 2016-2026 (US$ Mn)
9.4.5 Europe Radioimmunotherapy Market, by End-user, 2016-2026 (US$ Mn) 81
9.4.6 United Kingdom Country Profile
9.4.6.1 United Kingdom Radioimmunotherapy Market, 2016-2026 (US$ Mn)
9.4.7 Germany Country Profile
9.4.7.1 Germany Radioimmunotherapy Market, 2016-2026 (US$ Mn)
9.4.8 France Country Profile
9.4.8.1 France Radioimmunotherapy Market, 2016-2026 (US$ Mn)
9.4.9 Rest of Europe
9.4.9.1 Rest of Europe Radioimmunotherapy Market, 2016-2026 (US$ Mn)
9.5 Asia Pacific Radioimmunotherapy Market
9.5.1 Asia Pacific Radioimmunotherapy Market, by Drug Type, 2016-2026 (US$ Mn)
9.5.2 Asia Pacific Radioimmunotherapy Market, by Procedure Type, 2016-2026 (US$ Mn)
9.5.3 Asia Pacific Radioimmunotherapy Market, by Indication, 2016-2026 (US$ Mn)
9.5.4 Asia Pacific Radioimmunotherapy Market, by Radioisotope, 2016-2026 (US$ Mn)
9.5.5 Asia Pacific Radioimmunotherapy Market, by End-user, 2016-2026 (US$ Mn)
9.5.6 China Country Profile
9.5.6.1 China Radioimmunotherapy Market, 2016-2026 (US$ Mn)
9.5.7 Japan Country Profile
9.5.7.1 Japan Radioimmunotherapy Market, 2016-2026 (US$ Mn)
9.5.8 India Country Profile
9.5.8.1 India Radioimmunotherapy Market, 2016-2026 (US$ Mn)
9.5.9 Rest of Asia Pacific
9.5.9.1 Rest of Asia Pacific Radioimmunotherapy Market, 2016-2026 (US$ Mn)
9.6 Rest of the World Radioimmunotherapy Market
9.6.1 Rest of the World Radioimmunotherapy Market, by Drug Type, 2016-2026 (US$ Mn)
9.6.2 Rest of the World Radioimmunotherapy Market, by Procedure Type, 2016-2026 (US$ Mn)
9.6.3 Rest of the World Radioimmunotherapy Market, by Indication, 2016-2026 (US$ Mn)
9.6.4 Rest of the World Radioimmunotherapy Market, by Radioisotope, 2016-2026 (US$ Mn)
9.6.5 Rest of the World Radioimmunotherapy Market, by End-user, 2016-2026 (US$ Mn)
9.6.6 Latin America Country Profile
9.6.6.1 Latin America Radioimmunotherapy Market, 2016-2026 (US$ Mn)
9.6.7 Middle East & Africa Country Profile
9.6.7.1 Middle east & Africa Radioimmunotherapy Market, 2016-2026 (US$ Mn)
Chapter 10 Competitive Intelligence
10.1 Market Players Present in Market Life Cycle
10.2 Top 5 Players Comparison
10.3 Market Positioning of Key Players, 2018
10.4 Market Players Mapping
10.4.1 By Drug Type
10.4.2 By Procedure Type
10.4.1 By Indication
10.4.2 By Radioisotope
10.4.3 By End-user
10.4.4 By Region
10.5 Strategies Adopted by Key Market Players
10.6 Recent Developments in the Market
10.6.1 Mergers & Acquisitions, Partnership, New Product Developments
Chapter 11 Company Profiles
11.1 Acrotech Biopharma, LLC.
11.1.1 Acrotech Biopharma, LLC. Overview
11.1.2 Key Stakeholders/Person in Acrotech Biopharma, LLC.
11.1.3 Acrotech Biopharma, LLC. Products Portfolio
11.1.4 Acrotech Biopharma, LLC. Financial Overview
11.1.5 Acrotech Biopharma, LLC. News/Recent Developments
11.2 Actinium Pharmaceuticals, Inc.
11.2.1 Actinium Pharmaceuticals, Inc. Overview
11.2.2 Key Stakeholders/Person in Actinium Pharmaceuticals, Inc.
11.2.3 Actinium Pharmaceuticals, Inc. Products Portfolio
11.2.4 Actinium Pharmaceuticals, Inc. Financial Overview
11.2.5 Actinium Pharmaceuticals, Inc. News/Recent Developments
11.3 Advanced Accelerator Applications
11.3.1 Advanced Accelerator Applications Overview
11.3.2 Key Stakeholders/Person in Advanced Accelerator Applications
11.3.3 Advanced Accelerator Applications Products Portfolio
11.3.4 Advanced Accelerator Applications Financial Overview
11.3.5 Advanced Accelerator Applications News/Recent Developments
11.4 Bayer AG
11.4.1 Bayer AG Overview
11.4.2 Key Stakeholders/Person in Bayer AG
11.4.3 Bayer AG Products Portfolio
11.4.4 Bayer AG Financial Overview
11.4.5 Bayer AG News/Recent Developments
11.5 BioNTech SE.
11.5.1 BioNTech SE. Overview
11.5.2 Key Stakeholders/Person in BioNTech SE.
11.5.3 BioNTech SE. Products Portfolio
11.5.4 BioNTech SE. Financial Overview
11.5.5 BioNTech SE. News/Recent Developments
11.6 Immunomedics Inc.
11.6.1 Immunomedics Inc. Overview
11.6.2 Key Stakeholders/Person in Immunomedics Inc.
11.6.3 Immunomedics Inc. Products Portfolio
11.6.4 Immunomedics Inc. Financial Overview
11.6.5 Immunomedics Inc. News/Recent Developments
11.7 Nordic Nanovector
11.7.1 Nordic Nanovector Overview
11.7.2 Key Stakeholders/Person in Nordic Nanovector
11.7.3 Nordic Nanovector Products Portfolio
11.7.4 Nordic Nanovector Financial Overview
11.7.5 Nordic Nanovector News/Recent Developments
11.8 Oncoinvent AS
11.8.1 Oncoinvent AS Overview
11.8.2 Key Stakeholders/Person in Oncoinvent AS
11.8.3 Oncoinvent AS Products Portfolio
11.8.4 Oncoinvent AS Financial Overview
11.8.5 Oncoinvent AS News/Recent Developments
11.9 Orano Med
11.9.1 Orano Med Overview
11.9.2 Key Stakeholders/Person in Orano Med
11.9.3 Orano Med Products Portfolio
11.9.4 Orano Med Financial Overview
11.9.5 Orano Med News/Recent Developments
11.10 Telix Pharmaceuticals
11.10.1 Telix Pharmaceuticals Overview
11.10.2 Key Stakeholders/Person in Telix Pharmaceuticals
11.10.3 Telix Pharmaceuticals Products Portfolio
11.10.4 Telix Pharmaceuticals Financial Overview
11.10.5 Telix Pharmaceuticals News/Recent Developments