Canine stem cell therapy is a disease-modifying therapy used for the treatment of degenerative disorders such as arthritis of hips, elbows, shoulders, etc.
Canine Stem Cell Therapy Market Segmentation |
|
| By Type | 1. Autologous Stem Cells |
| 2. Allogeneic Stem Cells | |
| By Application | 1. Treatment |
| 2. Research | |
| By End-user | 1. Veterinary Hospitals |
| 2. Veterinary Clinics | |
| 3. Veterinary Research Institutes | |
| By Region | 1. North America (US and Canada) |
| 2. Europe (UK, Germany, France, Spain, Italy and Rest of Europe) | |
| 3. Asia Pacific (China, Japan, India and Rest of Asia Pacific) | |
| 4. Latin America (Brazil, Mexico and Rest of Latin America) | |
| 5. Middle East & Africa (GCC and Rest of Middle East & Africa) | |
Factors like rising prevalence of canine arthritis and increasing investment in research and development are expected to accentuate the growth of the canine stem cell therapy market. However, stem cell therapy is an expensive procedure as compared to other treatment options and this is impeding the growth of the market. Moreover, the lack of pet insurance will further restrain the growth of the market.

The Autologous Stem Cells segment is expected to dominate the market throughout the forecast period
Based on type, the global canine stem cell therapy market has been segmented into autologous stem cells and allogeneic stem cells. The autologous stem cells segment accounts for more than 80% of the global canine stem cell therapy market. This segment is expected to reach $xx million by 2026 from $xx in 2019, growing at a significant rate.
The major factor that is driving the growth of this segment is the approval of autologous stem cell therapy for indications such as spinal injuries, gastrointestinal issues, bone repair, and renal conditions. However, the expensive nature of the therapy as compared to allogeneic stem cell therapy and the longer time required for sample acquiring, processing, and the final treatment may impede the growth of this segment.
The allogeneic stem cell therapy segment is also growing at a significant rate. This segment has been driven by factors such as faster treatment availability, better efficacy rate, non-invasiveness of the therapy for patient animals, and cost benefits, as compared to the autologous stem cell therapy.
Based on application, the treatment segment is expected to dominant the market during the forecast period
Based on applications, the market has been segmented into treatment and research. The treatment segment accounts for the largest share in the global canine stem cell therapy market by application.
The rising prevalence of canine arthritis is driving the growth of this market segment. The increasing penetration of pet insurance and growing awareness about stem cell therapy will lead to the rising adoption of stem cell therapy and thereby, drive the treatment segment of the market.
Based on the end-user, the veterinary clinics segment is expected to dominate during the forecast period
Based on the end-user, the market has been segmented into veterinary hospitals, veterinary clinics, and veterinary research institutes. The veterinary clinics segment accounts for the largest share in the market and held more than 60% of the total market in 2018. The increasing number of veterinary clinics offering stem cell therapy will drive the growth of this end-user segment. Furthermore, there has been an expansion in the number of veterinary clinic chains, which has made treatment more accessible over a wide geography. This will also drive the growth of the veterinary clinics end-user segment.
North America to dominate the global canine stem cell therapy market throughout the forecast period
North America accounted for the highest share in 2018 and is expected to continue its dominance in the market throughout the forecast period. North America has the highest population of pet dogs, with around 73 million dogs in the US and over 6 million in Canada. The rising pet ownership in this region is one of the most important factors driving the growth of this market. The US accounted for around 90% of the market in 2018. The rising research and development activity in this region will also augment the growth of the canine stem cell therapy market.
Company Profiles and Competitive Intelligence:
The major players operating in this market are VetStem Biopharma, Medrego, and Magellan Stem Cells, amongst others. Most of the companies are conducting clinical trials to study the safety and efficacy of stem cells for various indication such as spinal disorders, renal disorders, etc.

Ask for free product review call with the author

Share your specific research requirements for a customized report
Request for due diligence and consumer centric studies

Request for study updates, segment specific and country level reports
Chapter 1 Preface
1.1 Report Description
1.1.1 Purpose of the Report
1.1.2 Target Audience
1.1.3 USP and Key Offerings
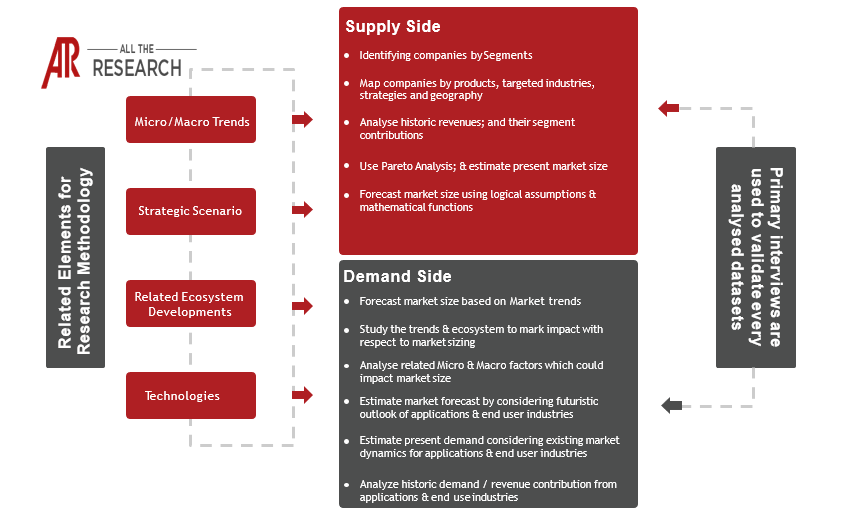
1.2 Research Scope
1.3 Research Methodology
1.3.1 Phase I – Secondary Research
1.3.2 Phase II – Primary Research
1.3.3 Phase III – Expert Panel Review
1.3.4 Approach Adopted
1.3.4.1 Top-Down Approach
1.3.4.2 Bottom-Up Approach
1.3.5 Assumptions
1.4 Market Segmentation Scope
Chapter 2 Executive Summary
2.1 Market Summary
2.1.1 Global Canine Stem Cell Therapy Market, 2016-2026, (US$ Mn)
2.1 Market Snapshot: Global Canine Stem Cell Therapy Market
2.2 Market Dynamics
2.3 Global Canine Stem Cell Therapy Market, by Segment, 2018
2.3.1 Global Canine Stem Cell Therapy Market, by Type, 2018, (US$ Mn)
2.3.2 Global Canine Stem Cell Therapy Market, by Application, 2018, (US$ Mn)
2.3.3 Global Canine Stem Cell Therapy Market, by End-user, 2018, (US$ Mn)
2.3.4 Global Canine Stem Cell Therapy Market, by Region, 2018 (US$ Mn)
2.4 Premium Insights
2.4.1 Canine Stem Cell Therapy Market In Developed Vs. Developing Economies, 2018 vs 2026
2.4.2 Global Canine Stem Cell Therapy Market: Regional Life Cycle Analysis
2.4.3 Industry Policies
2.4.4 Production Process and Manufacturing Cost Analysis
Chapter 3 Market Dynamics
3.1 Market Overview
3.2 Market Drivers
3.2.1 Increasing prevalence of chronic diseases
3.2.2 Increasing investment in R&D by market players and government bodies
3.3 Market Restraints
3.3.1 Expensive nature of the therapy
3.3.2 Restraint 2
3.4 Market Opportunities
3.4.1 Emerging applications of stem cell therapy
3.4.2 Opportunity 2
3.5 Industry Value Chain Analysis
3.5.1 Analyst’s Views
3.6 Industry SWOT Analysis
3.7 Industry Porter’s Five Forces Analysis
Chapter 4 Global Canine Stem Cell Therapy Market, by Type
4.1 Market Overview, by Type
4.1.1 Global Canine Stem Cell Therapy Market, by Type, 2016-2026 (US$ Mn)
4.1.2 Incremental Opportunity, by Type, From 2018-2026
4.2 Autologous Stem Cells
4.2.1 Global Canine Stem Cell Therapy Market, by Autologous Stem Cells, 2016-2026, (US$ Mn)
4.3 Allogeneic Stem Cells
4.3.1 Global Canine Stem Cell Therapy Market, by Allogeneic Stem Cells, 2016-2026, (US$ Mn)
Chapter 5 Global Canine Stem Cell Therapy Market, by Application
5.1 Market Overview, by Application
5.1.1 Global Canine Stem Cell Therapy Market, by Application, 2016-2026 (US$ Mn)
5.1.2 Incremental Opportunity, by Application, From 2018-2026
5.2 Treatment
5.2.1 Global Canine Stem Cell Therapy Market, by Treatment, 2016-2026, (US$ Mn)
5.3 Research
5.3.1 Global Canine Stem Cell Therapy Market, by Research, 2016-2026, (US$ Mn)
Chapter 6 Global Canine Stem Cell Therapy Market, by End-user
6.1 Market Overview, by End-user
6.1.1 Global Canine Stem Cell Therapy Market, by End-user, 2016-2026 (US$ Mn)
6.1.2 Incremental Opportunity, by End-user, From 2018-2026
6.2 Veterinary Hospitals
6.2.1 Global Canine Stem Cell Therapy Market, by Veterinary Hospitals, 2016-2026, (US$ Mn)
6.3 Veterinary Clinics
6.3.1 Global Canine Stem Cell Therapy Market, by Veterinary Clinics, 2016-2026, (US$ Mn)
6.4 Veterinary Research Institutes
6.4.1 Global Canine Stem Cell Therapy Market, by Veterinary Research Institutes, 2016-2026,
(US$ Mn)
Chapter 7 Global Canine Stem Cell Therapy Market, by Region
7.1 Market Overview, by Region
7.1.1 Global Canine Stem Cell Therapy Market, by Region, 2016-2026, (US$ Mn)
7.2 Attractive Investment Opportunity, by Region, 2018
7.3 North America Canine Stem Cell Therapy Market
7.3.1 North America Canine Stem Cell Therapy Market, by Type, 2016-2026 (US$ Mn)
7.3.2 North America Canine Stem Cell Therapy Market, by Application, 2016-2026 (US$ Mn)
7.3.3 North America Canine Stem Cell Therapy Market, by End-user, 2016-2026 (US$ Mn)
7.3.4 United States Country Profile
7.3.4.1 United States Canine Stem Cell Therapy Market, 2016-2026 (US$ Mn)
7.3.5 Canada Country Profile
7.3.5.1 Canada Canine Stem Cell Therapy Market, 2016-2026 (US$ Mn)
7.4 Europe Canine Stem Cell Therapy Market
7.4.1 Europe Canine Stem Cell Therapy Market, by Type, 2016-2026 (US$ Mn)
7.4.2 Europe Canine Stem Cell Therapy Market, by Application, 2016-2026 (US$ Mn)
7.4.3 Europe Canine Stem Cell Therapy Market, by End-user, 2016-2026 (US$ Mn)
7.4.4 United Kingdom Country Profile
7.4.4.1 United Kingdom Canine Stem Cell Therapy Market, 2016-2026 (US$ Mn)
7.4.5 Germany Country Profile
7.4.5.1 Germany Canine Stem Cell Therapy Market, 2016-2026 (US$ Mn)
7.4.6 France Country Profile
7.4.6.1 France Canine Stem Cell Therapy Market, 2016-2026 (US$ Mn)
7.4.7 Spain Country Profile
7.4.7.1 Spain Canine Stem Cell Therapy Market, 2016-2026 (US$ Mn)
7.4.8 Italy Country Profile
7.4.8.1 Italy Canine Stem Cell Therapy Market, 2016-2026 (US$ Mn)
7.4.9 Rest of Europe
7.4.9.1 Rest of Europe Canine Stem Cell Therapy Market, 2016-2026 (US$ Mn)
7.5 Asia Pacific Canine Stem Cell Therapy Market
7.5.1 Asia Pacific Canine Stem Cell Therapy Market, by Type, 2016-2026 (US$ Mn)
7.5.2 Asia Pacific Canine Stem Cell Therapy Market, by Application, 2016-2026 (US$ Mn)
7.5.3 Asia Pacific Canine Stem Cell Therapy Market, by End-user, 2016-2026 (US$ Mn)
7.5.4 China Country Profile
7.5.4.1 China Canine Stem Cell Therapy Market, 2016-2026 (US$ Mn)
7.5.5 Japan Country Profile
7.5.5.1 Japan Canine Stem Cell Therapy Market, 2016-2026 (US$ Mn)
7.5.6 India Country Profile
7.5.6.1 India Canine Stem Cell Therapy Market, 2016-2026 (US$ Mn)
7.5.7 Rest of Asia Pacific
7.5.7.1 Rest of Asia Pacific Canine Stem Cell Therapy Market, 2016-2026 (US$ Mn)
7.6 Latin America Canine Stem Cell Therapy Market
7.6.1 Latin America Canine Stem Cell Therapy Market, by Type, 2016-2026 (US$ Mn)
7.6.2 Latin America Canine Stem Cell Therapy Market, by Application, 2016-2026 (US$ Mn)
7.6.3 Latin America Canine Stem Cell Therapy Market, by End-user, 2016-2026 (US$ Mn)
7.6.4 Brazil Country Profile
7.6.4.1 Brazil Canine Stem Cell Therapy Market, 2016-2026 (US$ Mn)
7.6.5 Mexico Country Profile
7.6.5.1 Mexico Canine Stem Cell Therapy Market, 2016-2026 (US$ Mn)
7.6.6 Rest of Latin America
7.6.6.1 Rest of Latin America Canine Stem Cell Therapy Market, 2016-2026 (US$ Mn)
7.7 Middle East & Africa Canine Stem Cell Therapy Market
7.7.1 Middle East & Africa Canine Stem Cell Therapy Market, by Type, 2016-2026 (US$ Mn)
7.7.2 Middle East & Africa Canine Stem Cell Therapy Market, by Application, 2016-2026 (US$ Mn)
7.7.3 Middle East & Africa Canine Stem Cell Therapy Market, by End-user, 2016-2026 (US$ Mn)
7.7.4 GCC Country Profile
7.7.4.1 GCC Canine Stem Cell Therapy Market, 2016-2026 (US$ Mn)
7.7.5 Rest of Middle East & Africa
7.7.5.1 Rest of Middle East & Africa Canine Stem Cell Therapy Market, 2016-2026 (US$ Mn)
Chapter 8 Competitive Intelligence
8.1 Introduction
8.2 Players Evaluated During the Study
8.3 Market Players Present in Market Life Cycle
8.4 Top 5 Players Comparison
8.5 Market Positioning of Key Players, 2018
8.6 Market Players Mapping
8.6.1 By Type
8.6.2 By Application
8.6.3 By End-user
8.6.4 By Region
8.7 Strategies Adopted by Key Market Players
8.8 Recent Developments in the Market
8.8.1 Mergers & Acquisitions, Partnership, New Product Developments
8.9 Operational Efficiency Comparison by Key Players
Chapter 9 Company Profiles
9.1 VetStem Biopharma Inc.
9.1.1 VetStem Biopharma Inc. Overview
9.1.2 Key Stakeholders/Person in VetStem Biopharma Inc.
9.1.3 VetStem Biopharma Inc. Products Portfolio
9.1.4 VetStem Biopharma Inc. Financial Overview
9.1.5 VetStem Biopharma Inc. News/Recent Developments
9.2 Cell Therapy Sciences
9.2.1 Cell Therapy Sciences Overview
9.2.2 Key Stakeholders/Person in Cell Therapy Sciences
9.2.3 Cell Therapy Sciences Products Portfolio
9.2.4 Cell Therapy Sciences Financial Overview
9.2.5 Cell Therapy Sciences News/Recent Developments
9.3 Aratana Therapeutics, Inc.
9.3.1 Aratana Therapeutics, Inc. Overview
9.3.2 Key Stakeholders/Person in Aratana Therapeutics, Inc.
9.3.3 Aratana Therapeutics, Inc. Products Portfolio
9.3.4 Aratana Therapeutics, Inc. Financial Overview
9.3.5 Aratana Therapeutics, Inc. News/Recent Developments
9.4 Regeneus Ltd
9.4.1 Regeneus Ltd Overview
9.4.2 Key Stakeholders/Person in Regeneus Ltd
9.4.3 Regeneus Ltd Products Portfolio
9.4.4 Regeneus Ltd Financial Overview
9.4.5 Regeneus Ltd News/Recent Developments
9.5 Medivet Biologics LLC
9.5.1 Medivet Biologics LLC Overview
9.5.2 Key Stakeholders/Person in Medivet Biologics LLC
9.5.3 Medivet Biologics LLC Products Portfolio
9.5.4 Medivet Biologics LLC Financial Overview
9.5.5 Medivet Biologics LLC News/Recent Developments
9.6 Vetbiologics
9.6.1 Vetbiologics Overview
9.6.2 Key Stakeholders/Person in Vetbiologics
9.6.3 Vetbiologics Products Portfolio
9.6.4 Vetbiologics Financial Overview
9.6.5 Vetbiologics News/Recent Developments
9.7 Stemcellvet
9.7.1 Stemcellvet Overview
9.7.2 Key Stakeholders/Person in Stemcellvet
9.7.3 Stemcellvet Products Portfolio
9.7.4 Stemcellvet Financial Overview
9.7.5 Stemcellvet News/Recent Developments
9.8 Magellan Stem Cells
9.8.1 Magellan Stem Cells Overview
9.8.2 Key Stakeholders/Person in Magellan Stem Cells
9.8.3 Magellan Stem Cells Products Portfolio
9.8.4 Magellan Stem Cells Financial Overview
9.8.5 Magellan Stem Cells News/Recent Developments
9.9 Medrego
9.9.1 Medrego Overview
9.9.2 Key Stakeholders/Person in Medrego
9.9.3 Medrego Products Portfolio
9.9.4 Medrego Financial Overview
9.9.5 Medrego News/Recent Developments